Innovation at Prosit Sole

At Prosit Sole, the four highly experienced executives of the core R&D management team brings with them outstanding scientific background and strong executive experiences. Each has over 25 years of pharmaceutical industrial experiences and/or extensive track records of successfully discovering, developing multiple first-in-class biologics with large and durable commercial potentials. Some of them are long-time professional associates and/or acquaintances with shared value and aspiration of developing life-saving and/or life–enhancing medicines for patient with rare diseases at an ever accelerated pace. The combined over 100 years of industry experiences positions us very well to maximize the potential of our deep understanding of disease biology and drug discovery platform.
Prosite Soleis the proud inventor and owner of an patented biologics discovery platform and a robust product pipeline, with 6 innovative therapeutics for treatment of a range of severe rare diseases. Each of biologics molecule is designed to be de-risked as well as likely to be speedily approvable by FDA and CFDA. Prosite Sole has a number of industry veterans and experts in various functions on its R&D staff from discovery, translational sciences, early development to clinical trials. The company possesses full capability of protein design by the patented P-POP protein chimeric platform, drug screening, cell banking, high quality scale-up/manufacturing, animal disease models, preclinical and clinical development.

Molecule Design
Prosit Sole Focuses on Un-met medical need
Novel therapeutic targets leaded by new scientific discovery
Through Innovative Protein Chimeric Technology
How to Guarantee Quality at Prosit Sole?
At Prosit Sole, The three level cell banks were strickly constracted under GMP equivalent condition in pilot facility. All cell banks passed through stability evaluation. The result shows, that after 100 generations of continuous cultivation, the plasmid retention rate is more than 95%, which met the criteria for GMP production.
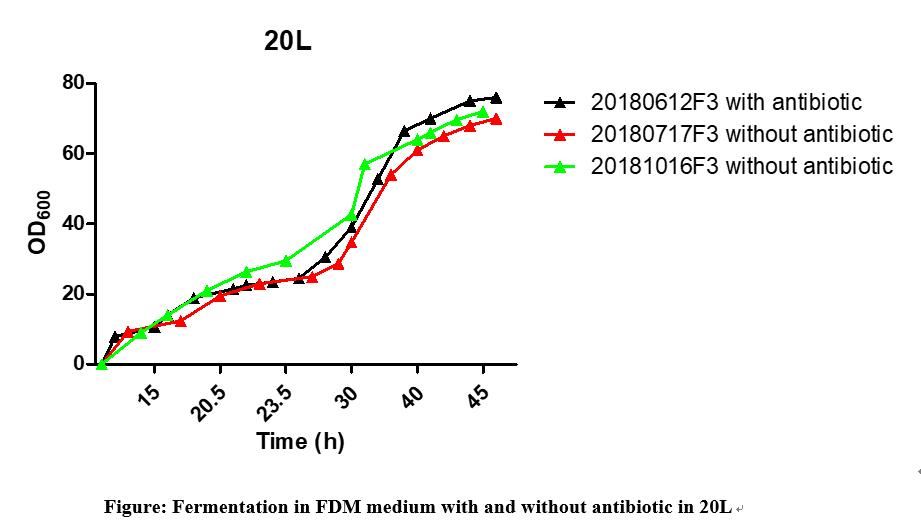
Optimization of FermentationProcess:Considering the safety, Prosit Sole develops a completely inorganic salt based culture medium and antibiotic-free fermentation process. The overall control SOP of seed recovery, seed expansion and fermentation were optimized according to FDA and CFDA’s requirment. The production process is antibiotic free.
Molecure Screening and Optimizing
Interestingly, all four analogs showed potent activity in inducing the expression of the antiviral genes MxA and OAS and two of them, analog-6 and -7, displayed an unexpected high potency that is higher than that of type I IFN (IFN-α2a) . Together, these studies identified two IFN-λ analogs as candidates to be developed as novel antiviral biologics. Keywords: type III interferon, IFN-λ, IL-29, IL-28B, analog, antiviral