干扰素λ嵌合体
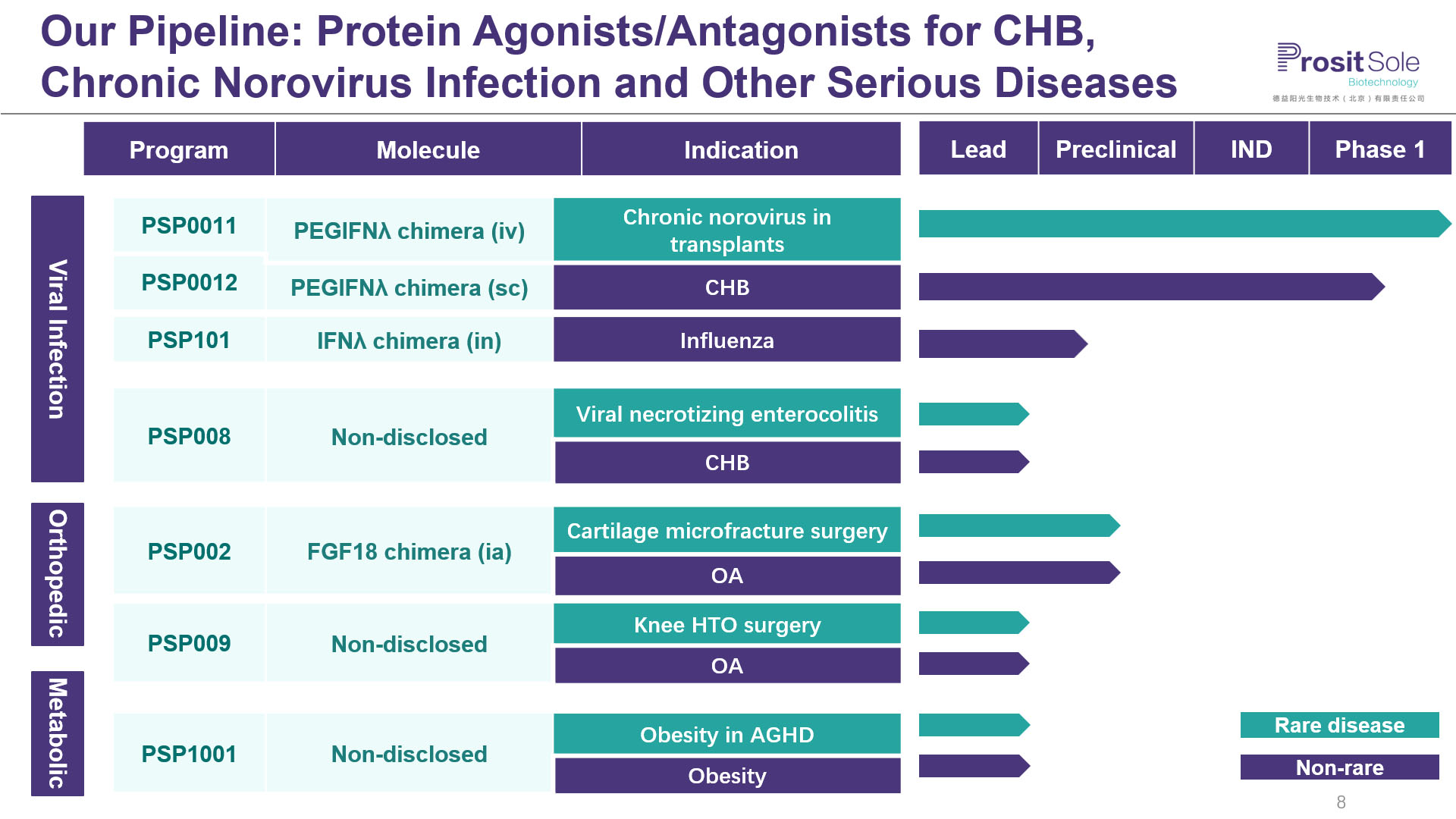
Pipeline of Prosit Sole
A New INFλ Chimera -- a critical players in the defense against viral infection with improved efficacy and safety profile
Prosit Sole Biotechnology (Beijing) Co Ltd. (PSB) discovered and is developing a novel protein therapeutic agent, PSP001, for the treatment of chronic norovirus infection in patients who have undergone allograft kidney transplantation. PEGinterferon λ1-λ3 Chimera, the active pharmaceutical ingredient of PSP001, has been fully characterized during manufacturing process development conducted at Prosit Sole. This product has shown consistent identity, purity, potency and stability as well as biological activity against the virus of interest. Additionally, research as reported in the published literature supports its clinical potential (Yu, et al. 2016).
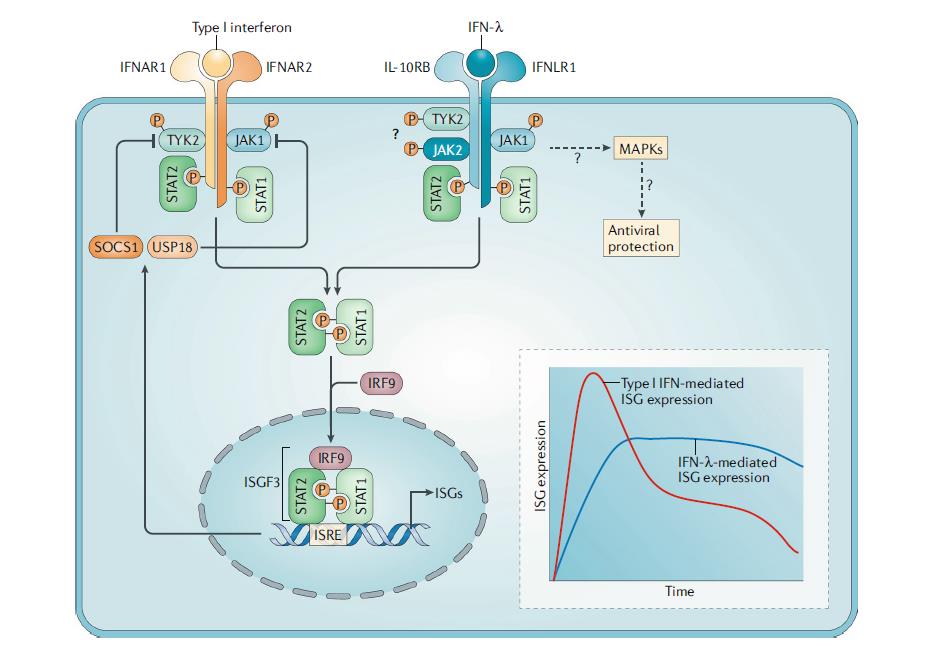
Type III interferons (interferon λ1, λ2, λ3, and λ4) are critical players in the defense against viral infection of mucosal epithelial tissues, where the activity of type I IFNs is weak (Pott, et al. 2011). In a mouse model of murine norovirus, interferon λ treatment cleared persistent viral infection in the absence of adaptive immunity (Nice, et al. 2015). The efficacy and safety of PEGinterferon λ1a have been evaluated in patients with HBV and HCV in clinical trials (Chan, et al. 2016; Foster, et al. 2016). Compared to PEGinterferon α2a, PEGinterferon λ1a exhibits comparable or slightly less antiviral activity against these viruses with a differentiated but mostly favorable safety profile. As such, type III interferons appear to be promising agents for the treatment and prevention of gastrointestinal viral infections (Li, et al. 2009).
Life-threatening Nature of Norovirus Infection in Immune-Compromised Individuals

Human noroviruses (HuNoVs), with a single-stranded positive-sense RNA genome, belong to the family Caliciviridae. There are six major genogroups (Genogroup I through Genogroup VI, GI - GVI). GI and GII contain the majority of norovirus strains associated with human disease (Bok, et al. 2012). In the United States, HuNoVs cause approximately 23 million infections per year (Sarvestani, et al. 2016). Clinical symptoms (diarrhea, vomiting, nausea, and abdominal cramps) are generally self-limiting and resolved in 1-3 days in immune-competent patients. However, in immune-compromised individuals, including allograft recipients under immunosuppressant treatment for Graft vs. Host Disease, HuNoVs infections can result in prolonged illness and even death (Robles, et al. 2012; Schorn, et al. 2010). Currently there is no approved HuNoV specific treatment.
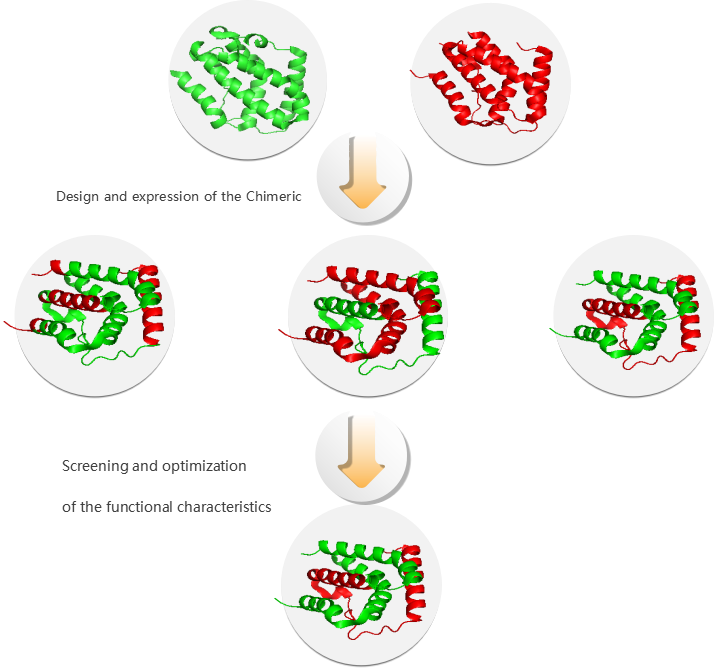
Design of INF-λ Chimera